
How AcellFX can make a difference
AcellFX aids restoration of the ocular surface
- AcellFX provides a protective environment for repair to the cornea and conjunctiva*
- AcellFX is processed to be an acellular, immune-privileged, human amniotic membrane4
- Amnion contains biofactors such as collagen III, IV, and V; fibronectin; laminin; and proteoglycans5
Easy to use6:
Ready for immediate use
No thawing or rinsing necessary
Room temperature–stable for 5 years, allowing for convenient storage
Allows for multiple application techniques
There is no up or down orientation
CPT CODE 65778: Placement of amniotic membrane on the ocular surface without sutures†

Many conditions damage the ocular surface
Conditions could include‡:
- Keratitis, including neurotrophic keratitis1
- Severe dry eye disease3
- Corneal ulcers7
- Chemical trauma1
- Bullous keratopathy7
- Corneal degeneration1
- Recurrent epithelial erosion3
- Physical trauma1
- Chronic Stevens-Johnson syndrome or Lyell’s syndrome8,9
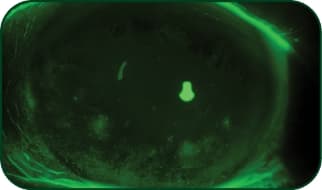
Superficial punctate keratitis (SPK)
Photo: Etty Bitton, OD, MSc, FAAO
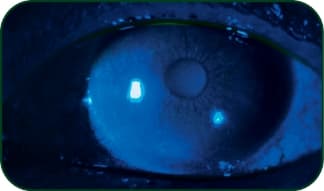
Keratoconjunctivitis sicca (KCS)
Photo: Stephen Pfugfelder, MD
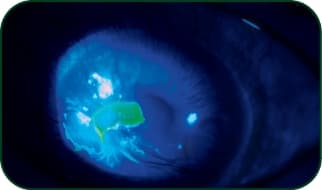
Neurotrophic keratitis (NK)
Photo: Stephen Pfugfelder, MD
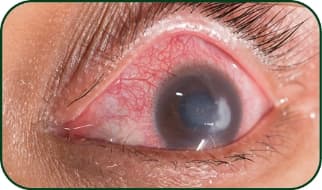
Corneal ulcer
AcellFX is designed for patient comfort

- AcellFX is a high-quality human membrane that is acellular and immune-privileged4
- Gently processed without harsh chemicals, such as cross-linking agents, germicides, or antibiotics6
- Air-dried, not chemically dried
- Sterilized to a sterility assurance level of 10-6
- No ring required and can be placed outside the visual axis
Download AcellFX Patient Brochure

The power of AcellFX processing
AcellFX is air-dried and ready for the eye
With the removal of the epithelium and chorion layers of the amniotic membrane, AcellFX is processed to be acellular.
- May lower risk of rejection by host (immune response)
- Supports ease of use in that there is no up or down orientation


References: 1. Walkden A. Amniotic membrane transplantation in ophthalmology: an updated perspective. Clin Ophthalmol. 2020;14:2057-2072. 2. Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15(3):276-283. 3. Jones L, Downie LE, Korb D, et al. TFOS DEWS II management and therapy report. Ocul Surf. 2017;15(3):575-628. 4. Magatti M, Vertua E, Cargnoni A, Silini A, Parolini O. The immunomodulatory properties of amniotic cells: the two sides of the coin. Cell Transplant. 2018;27(1):31-44. 5. Niknejad H, Peirovi H, Jorjani M, Ahmadiani A, Ghanavi J, Seifalian AM. Properties of the amniotic membrane for potential use in tissue engineering. Eur Cell Mater. 2008;15:88-99. 6. AcellFX. Package insert. Thea Pharma, Inc; 2021. 7. Jirsova K, Jones GLA. Amniotic membrane in ophthalmology: properties, preparation, storage and indications for grafting—a review. Cell Tissue Bank. 2017;18(2):193-204. 8. Dua HS, Gomes JAP, King AJ, Maharajan VS. The amniotic membrane in ophthalmology. Surv Ophthalmol. 2004;49(1):51-77. 9. Sangwan VS, Burman S, Tejwani S, Mahesh SP, Murthy R. Amniotic membrane transplantation: a review of current indications in the management of ophthalmic disorders. Indian J Ophthalmol. 2007;55(4):251-260.
*There are no specific FDA indications for the product.
†This information does not guarantee payment and is not billing or coding advice. Providers are responsible for determining appropriate coding and submission of accurate claims.
‡THE CONDITIONS LISTED ARE FOR REFERENCE PURPOSES ONLY.
Before use, please refer to Information For Use (IFU) package insert.


